Text: Senne Starckx/Photos: Saskia Vanderstichele
Uncharted territory
Structural biology is the study of precisely these building blocks, which combine to form complex molecules such as proteins and DNA at a higher level. The focus is on the ways in which the thousands of proteins form spatial structures rather than on the complex biomolecule composition, which is no longer the mystery it once was. The three-dimensional structures studied in structural biology determine how a protein functions, the way in which other, often much smaller molecules bind to it - and their influence on how the protein functions.
“This was largely uncharted territory when I was a student,” Steyaert says. “We’ve come a long way since then, but I continue to feel as if the next discovery is waiting for us right around the corner.” In most experimental sciences, new discoveries are often the result of improved research equipment, and structural biology is no exception. It’s why the inauguration of the cryo-electron microscope will mark the beginning of a new era, one in which biologists like Steyaert will be able to uncover a wealth of new molecular structures.
These new findings could be applied in countless ways. They might restart stalled drug discovery projects and be used by pharmaceutical researchers to develop new medicines. Until recently, this search for new medicines was a trial-and-error process. Structural biology makes it possible to target precisely those proteins of which the structure is already known. This will exponentially increase the chance of successful pharmaceutical discoveries and shorten the time it takes to develop new drugs.
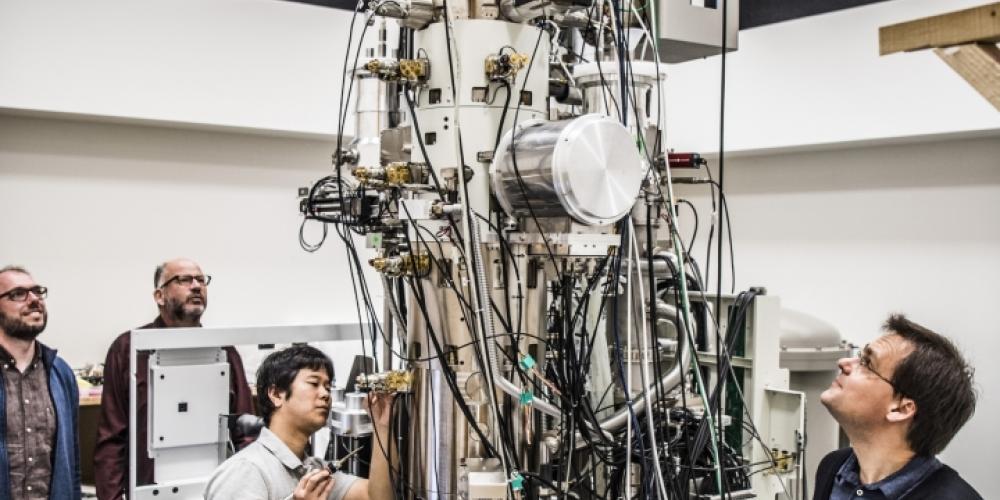
Story continues under picture.
You’d be surprised how little we know about the drugs we use.
You’d be surprised how little we know about the drugs we use. Take diazepam for instance. We’ve known which protein this sleeping and anti-anxiety drug targets for decades. But we didn’t know where or how exactly Valium binds to the protein receptor.
The Valium riddle
Cryo-electron microscopes from the universities of Oxford and Cambridge helped solve this Valium riddle. A cryo-electron microscope freezes a purified protein sample into an incredibly thin layer of ice and subsequently places it under a highly sensitive microscope. One sample contains millions of specimens of the same protein, each frozen in a different orientation. Because of the molecules’ random orientation, the electron microscope makes images of all the projections. In other words, the microscope doesn’t turn round; the proteins instead show all their sides. “The electrons penetrate straight through the molecular structure and can only be stopped by colliding with an atom,” Steyaert explains. “By detecting individual electrons that aren’t absorbed, we get a two-dimensional projection of the three-dimensional protein structure.”
The resolution of such separate projections is somewhat disappointing. “But we have thousands of images for each orientation,” Steyaert says, and because the molecules are identical, it doesn’t matter that the images are of different molecules. “By combining these images with the computer, we obtain two-dimensional images at a higher resolution. We subsequently repeat this process for each orientation and then combine these very sharp images into a three-dimensional image. This way we can reveal the protein’s complete structure in 3D.”
Breakthrough research
The genius of the cryo-electron microscope hasn’t escaped the notice of the Nobel Prize committee. Last year, Jacques Dubochet, Joachim Frank and Richard Henderson received the Nobel Prize in Chemistry for their work, which the committee hailed as a “breakthrough in the study of live, active proteins.” Henderson will attend the inauguration of the cryo-electron microscope in September.
Until recently, the structure of proteins could only be determined through X-ray crystallography, which requires molecules to be in a solid form, or in other words to have been crystallised. Unfortunately, this is impossible with many human, animal and bacterial proteins.
The three Nobel Prize winners exchanged the X- rays for particles – or electrons – in order to inspect the proteins in a solution, a setting that strongly resembles their natural environment. For a long time, the resolution of images made with the previously used electron microscopes was much too low to be able to reconstruct the three-dimensional structure at the atomic level. It’s why structural biologists jokingly started to refer to this as the ‘blobology’ method – a hazy, slimy blob was after all all you got.
Dubochet, Frank and Henderson eventually managed to increase the electron microscope’s resolution. As the ‘cryo’ in the name of the new microscope suggests, they also developed freezing methods and computer software to combine the images of the protein’s different orientations into a 3D structure.
Story continues under picture.
Only two in the world
The cryo-electron microscope being installed in former on-campus student housing is only the second of its generation – the other is in Japan. That means Brussels and the VUB campus will become a European and global hub for cryo-electron microscopy in a couple of months. “The idea is that the microscope will be used twenty-four hours a day, seven days a week from September,” says Steyaert. “We will use it seventy per cent of the time; the remaining time will go toward Flemish and European scientists as well as industry researchers.”
The pharmaceutical industry is especially keen to work with the new microscope as detailed knowledge of human and other protein structures is crucial to the discovery and continued development of new active substances. But what happens when the structure of a protein has been mapped? “We have embraced the open science principle in structural biology for years,” Steyaert says. “Whenever a structure is published, it automatically goes into the Protein Data Bank, a central database that can be accessed by every researcher in this discipline.”
The VUB researchers also hope to be able to forge their own breakthrough with the brand-new cryo-electron microscope before too long. “We’re developing technology to make images over time and to translate these into ‘real-life’ clips of active proteins or complete cell parts.” But before that happens, Steyaert and his colleagues will be concentrating their efforts on the innumerable proteins that don’t crystallise. With the new cryo-electron microscope, they’ll be able to finally uncover their structure. “We’ll soon be able to see structures that have never before been mapped. It’s terribly exciting; we are entering a brave new world.”